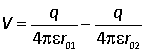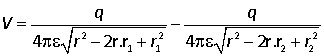Level 1 (gold) - this material has some prerequisites that are covered in the first year mathematics for chemists course. In particular we will use the binomial expansion, vectors and simple partial differentiation.
A neutral molecule may experience eletrical forces even though it is electrically neutral, because the charges are distributed asymetrically some way within the molecule. For example in a simple system of two equal and opposite charges, a third charge would experience a force as indicated by the field lines discussed in the section on electric fields. And if this charge distribution were introduced into an external electric field it would experience a force as a result. |
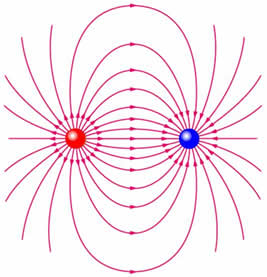 |
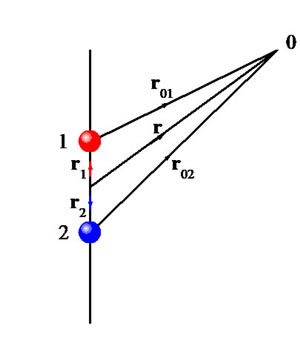
We introduce the dipole moment through the simple system of two equal and opposite charges in the figure above, and we will calculate the potential and electric field around this charge distribution. This is then generalised to an arbitrary number of charges.
Suppose that a system contains two charges, +q at position vector r1, and –q at position r2. Consider the potential at a test point 0 at r. This depends on the distances from the two charges to the test point, r01 and r02. These distances may be found from the vector diagram opposite. For example Hence Thus
|
 |
No further simplification can be made except when the test point is far from the charge distribution. In this case the first order potential is zero because the contributions from the equal and opposite charges almost cancel out. However there will be a second order contribution because the distances to the two charges are different.
We therefore consider the case where r is much larger than r1 and r2 and factor out r.
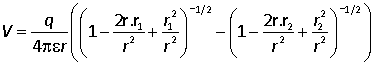
We are only concerned with the leading term in the potential, and each of the terms contains a term of order 1, a term of order r1/r and a term of order (r1/r)2. The final term can therefore be neglected:
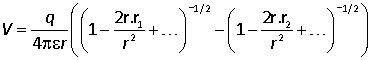
Now expand the two brackets using the binomial expansion:
![]()
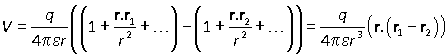
The terms neglected in the expansion are of the same order (or smaller) than those already removed. Notice that the constant terms cancel one another, because to a first approximation the potentials from interaction with each charge are equal and opposite.
The vector r1–r2 is the interparticle vector r12. Defining the dipole moment vector p to equal qr12, the result can be written
![]()
The same method can be applied to a larger number of charges. Suppose that particle i has charge qi and position vector ri:
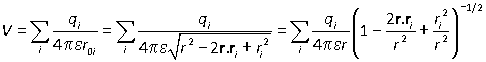
and in the far field limit
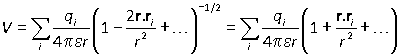
Since the charge distribution is neutral overall
![]()
and in consequence
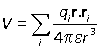
Defining the dipole moment as
![]()
the potential is given again by
![]()
(1) The special case of two equal and opposite charges is equivalent to the case dealt with earlier:
![]()
(2) The dipole moment of a neutral molecule is independent of the origin:
Referring all vectors to a new origin r0,
![]()
because
![]()
(3) Dipole moment of a molecule.
The sum covers nuclei and electrons.
Since the electrons move much more rapidly than the nuclei, the electron charge can be averaged. The dipole is often modelled by assigning partial charges to the atoms.
Example
atom |
x/Å |
y/Å |
z/Å |
q/e |
O |
0 |
0 |
+.1173 |
–.6568 |
H |
0 |
+.7572 |
–.4692 |
+.3284 |
H |
0 |
–.7572 |
–.4692 |
+.3284 |
Dipole moment is (0, 0, –0.3852) Å e = (0, 0, –6.171×10–30) C m The magnitude is 6.171×10–30 C m = 1.850 D
The nonstandard unit Debye (D) is often used for molecular dipole moments. It is defined by 1 D = 3.33564×10–30 C m (Debye)
Electric field around a dipole
The electric field in the far field region can be found from the potential by calculating the gradient of the potential (see section on potential):
E = –∇V
that is
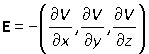
Returning to the result of the previous section
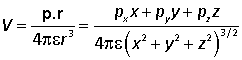
we differentiate with respect to x
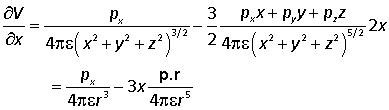
with similar results for the y and z derivatives.
Hence
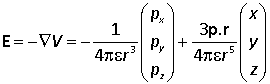
or in vector notation
![]()
using the ^ to denote a unit vector.
Consider a two charge dipole centred at the origin in a constant electric field E oriented along the z direction. The charges are separated by a distance d and the dipole makes an angle θ with the field. The z coordinates of the two charges are Hence (see section potential) the energy of the dipole is In the general case the interaction energy is The minimum energy is the case where θ = 0, the dipole is lined up antiparallel to the field, and the maximum energy is where θ = π, the dipole is parallel to the field. |
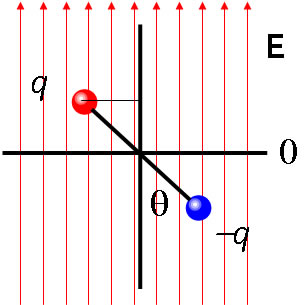 |
The dipole will also experience a force as a result of its orientation in the field. In the two charge case the force on particle 1 is qE in the z direction and the force on particle 2 is equal and opposite. The force on charge 1 can be resolved into components parallel to and perpendicular to the dipole moment. The parallel component is qE cos θ and distorts the molecule by stretching. The perpendicular component is a torque which turns the molecule towards its minimum energy orientation in the field. |
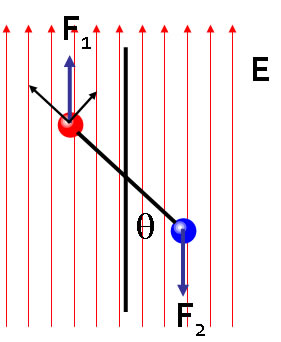 |
Charge dipole interaction energy
The interaction between a charge and a dipole is simple to calculate. The potential of a dipole is given by
![]()
and therefore the energy of a test charge q at position r relative to the centre of the dipole is
![]()
Charge-dipole force
Similarly the electric field around the dipole is
![]()
and so the force on the charge is
![]()
Dipole-dipole interaction energy
The interaction energy between two dipoles can be found by considering the energy of one dipole in the field produced by the other one. So consider a dipole p1 at the origin and a dipole p2 at position r12. The field experienced by dipole 2 is therefore
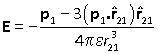
where the unit vector is in the direction of dipole 2 from dipole 1
The energy of interaction of dipole 2 with the field is therefore
![]()
which is
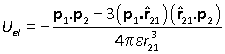
Notice two important points about this interaction.
(1) The energy is inversely proportional to the cube of the distance between the dipoles (cf charge-charge energy r–1 and charge-dipole energy r–2).
(2) The interaction is symmetric between the two dipoles, i.e. the interaction of 2 with the field of 1 is identical to the interaction of 1 with the field of 2.