Page 16 - PERIODIC Magazine Issue 5
P. 16
Mo lecular Movies
Researchers Michael Burt, Alexander Gentleman and Jason Lee
explain how the ultra-fast PImMs camera enables scientists to watch
chemistry as it happens.
Our world is composed of molecules that constantly valuable information about how the energy deposited into
interact, and this interplay controls everything from the a molecule is redistributed over time. This redistribution
behaviour of the atmosphere to the biology in every can cause the molecule to change shape, become
cell. The Nobel-Prize-winning field of reaction dynamics vibrationally and electronically excited, or to break up
investigates chemical interactions at the atomic level with into smaller fragments. Observing these processes sheds
the aim of explaining, and eventually controlling, how light on the underlying chemistry and directly probes the
chemical reactions take place. Over the past few years, “energy landscape” of the molecule, which defines all of
the research groups of Mark Brouard, Stuart Mackenzie, the molecular forces and behaviour, and in particular their
and Claire Vallance (University of Oxford) have been likelihood to undergo particular reactions. The specialised
working with the groups of Mike Ashfold, Jeremy Harvey, electronics in the PImMS sensor allow it to operate at
and Andrew Orr-Ewing (University of Bristol) as part of an 80 million frames per second, fast enough to separate
EPSRC Programme Grant that explores the dynamics of chemical fragments based on their mass, and to watch
new chemical systems and develops novel experiments chemistry as it occurs.
that push the boundaries of this field.
In combination with ultra-fast laser pulses, the PImMS
One promising research direction involves the sensor can be used to investigate molecular structures.
development of a new sensor capable of watching Intense radiation can ionise multiple electrons from
chemistry in action. Scientists from Oxford Chemistry, a molecule, removing the bonds holding the nuclei
Oxford Physics and the Rutherford Appleton Laboratory together. Repulsion between the positively charged nuclei
have combined their expertise to create the Pixel Imaging causes the molecular ion to explode into fragments with
Mass Spectrometry (PImMS) sensor, which provides trajectories that are governed by Coulomb’s law. If the
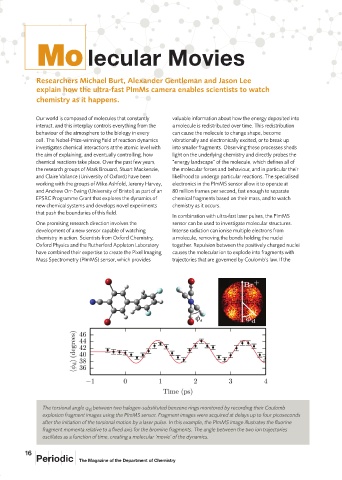
The torsional angle between two halogen-substituted benzene rings monitored by recording their Coulomb
d
explosion fragment images using the PImMS sensor. Fragment images were acquired at delays up to four picoseconds
after the initiation of the torsional motion by a laser pulse. In this example, the PImMS image illustrates the fluorine
fragment momenta relative to a fixed axis for the bromine fragments. The angle between the two ion trajectories
oscillates as a function of time, creating a molecular ‘movie’ of the dynamics.
16
Periodic The Magazine of the Department of Chemistry